Featured Results
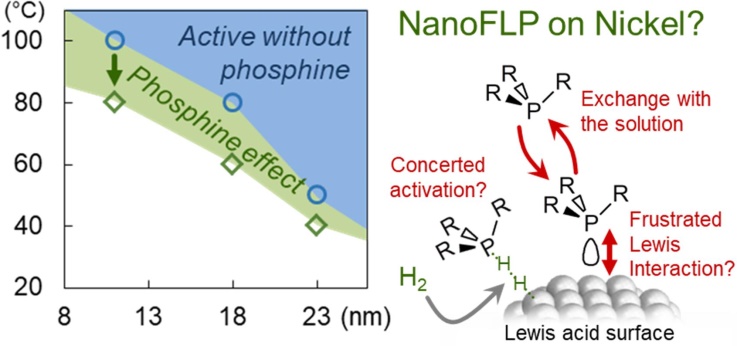
Refining the NanoFLP ConceptStill fighting to unveil an authentic NanoFLP, we uncovered an interesting low-temperature behavior of Ni nanoparticles in the presence of well-selected phosphine. This could be interpreted as the formation of a surface frustrated Lewis pair for H-H cleavage... but also as a consequence of phenylacetylene activation. What do you make of this? |
 |
Carbon is our friend!Nickel fame for catalysis now extends to a new guy: nickel carbide. In this work, we show that this phase can be used as a catalyst for the hydrgenation of a number of moieties, coming along a greater robustness than the metallic counter-part. Check out our nickel carbide buddy. |
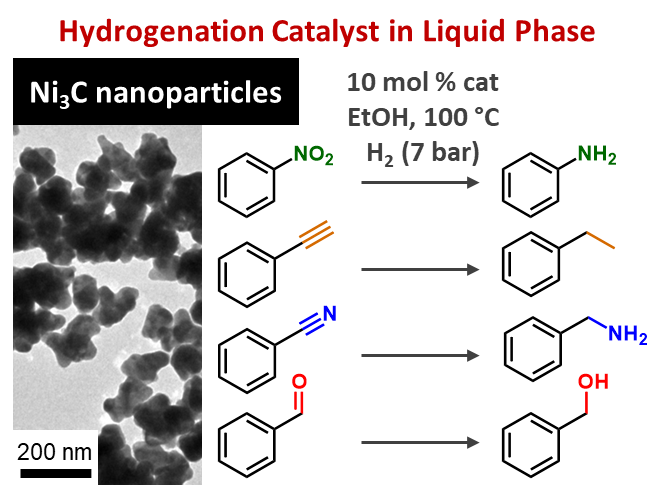 |
Make them saferIt's a small step for the world of nanoparticles, but a big step for our beloved oxysulfides nanoplates: in this work, we propose to evaluate, in a very preliminary approach, some of the interactions of the nanoparticles with macrophage cells. We turned on the lamp (visible light) and monitored the impact in the production of radicals. Read for yourself. |
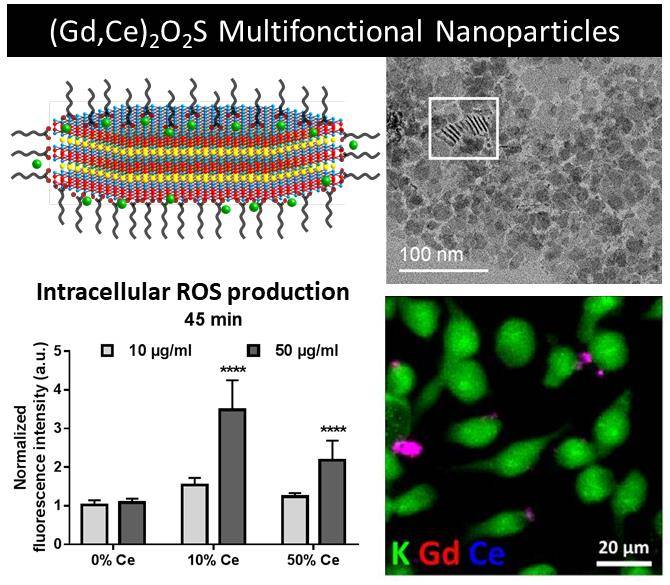 |
Did you say NanoFLP?We have been proposing for a while to use metal nanoparticles as partners in Frustrated Lewis Pairs. Now, we found that nickel-cobalt nanoparticles associated to well-chosen phosphines likely form such a pair, based on the correlation between Si-H bond activation and the Tolman cone angle of the phosphine: only a tiny range of steric hindrance is suitable, and it is related to the silane bulkiness. We believe we nailed it. Make your own opinion. |
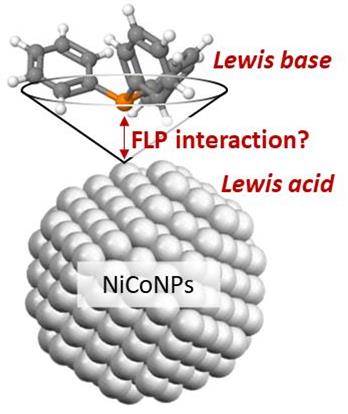 |
Molybdenum mapped outMolybdenum is a key element of the energy transition. X-ray absortion spectroscopy at the L-edge is an amazing way to analyze it in solids, liquids, composites, etc. Here, we wrote some simple guidelines to interpret the data and we provided a consistent series of spectra for well-known, and less-know, Mo compounds. Read our map. |
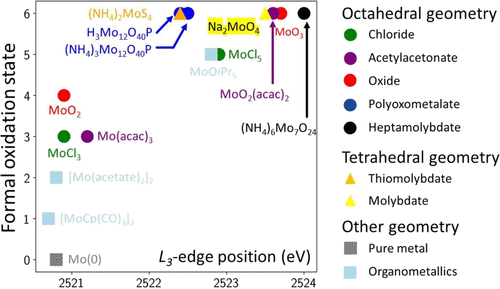 |
Green deal on nanocarbidesHere is a dream reaction: no solvent, no waste (except salt), no heat input. Check out how we produced a variety of metal carbide nanoparticles supported on graphite or acetylene black. We also discuss secondary formation of hydrides, metals and oxides using a simple adiabatic approximation. |
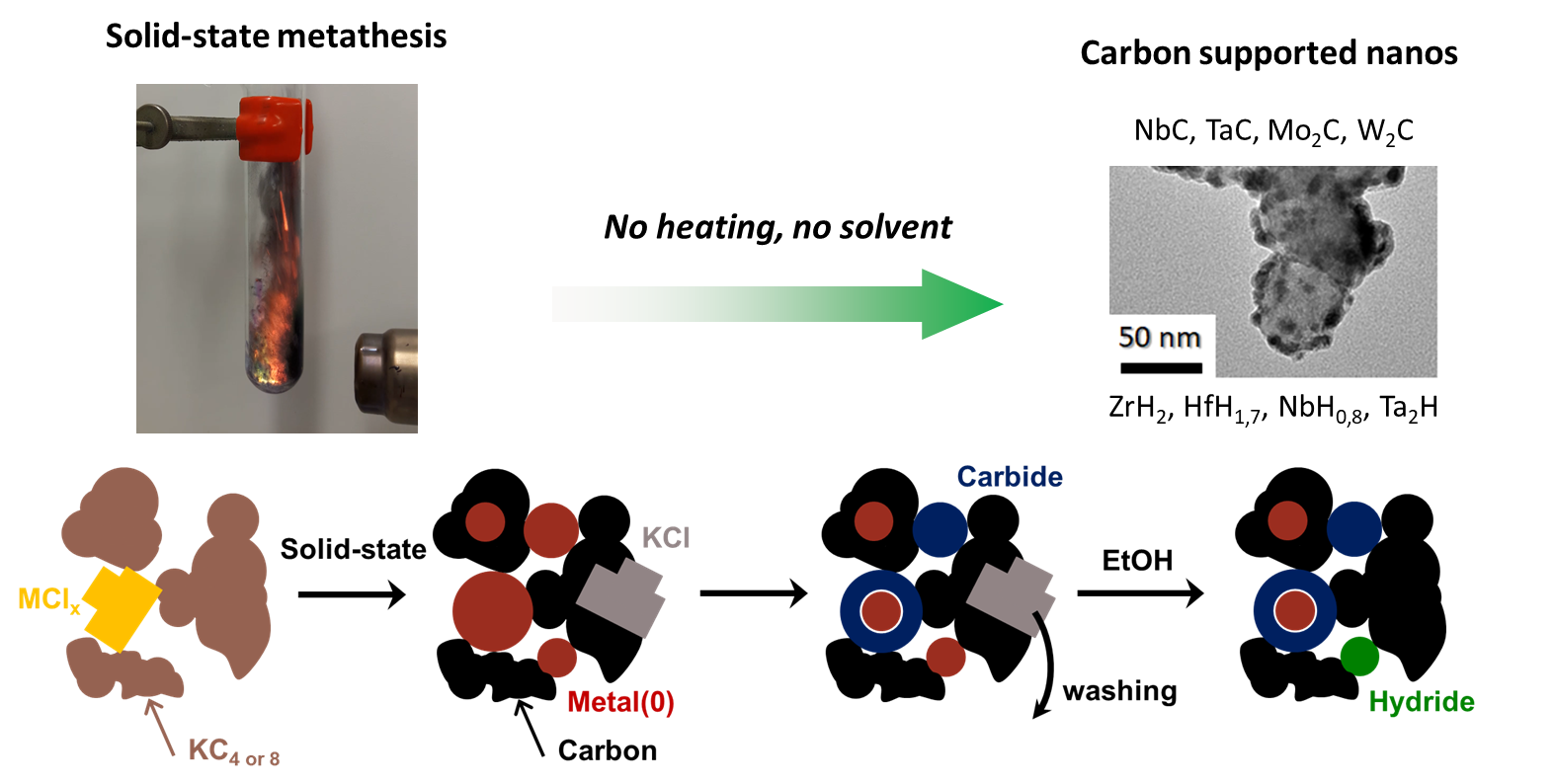 |
Water mattersWe revisited copper nanoparticles synthesis and showed that, depending on the precursor, the amount of water produced in situ is slightly different. This is enough to modulate the catalytic processes that consume the solvent and reducing agent of the reaction: oleylamine. Read how and why. |
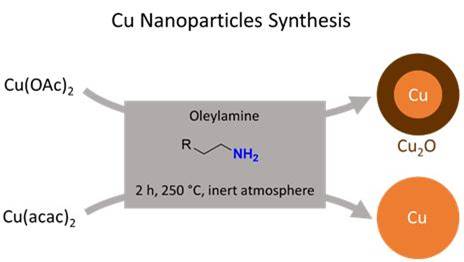 |
Amorphous nanoparticles on the spotlightMany works focus on the electrocatalytic performances of crystallline metal phosphide nanoparticles. Using a new phosphorus donor, we produced amorphous FeP, we showed that the local structure is similar to these of the crystal, and we found it is more active for HER. Check it out. |
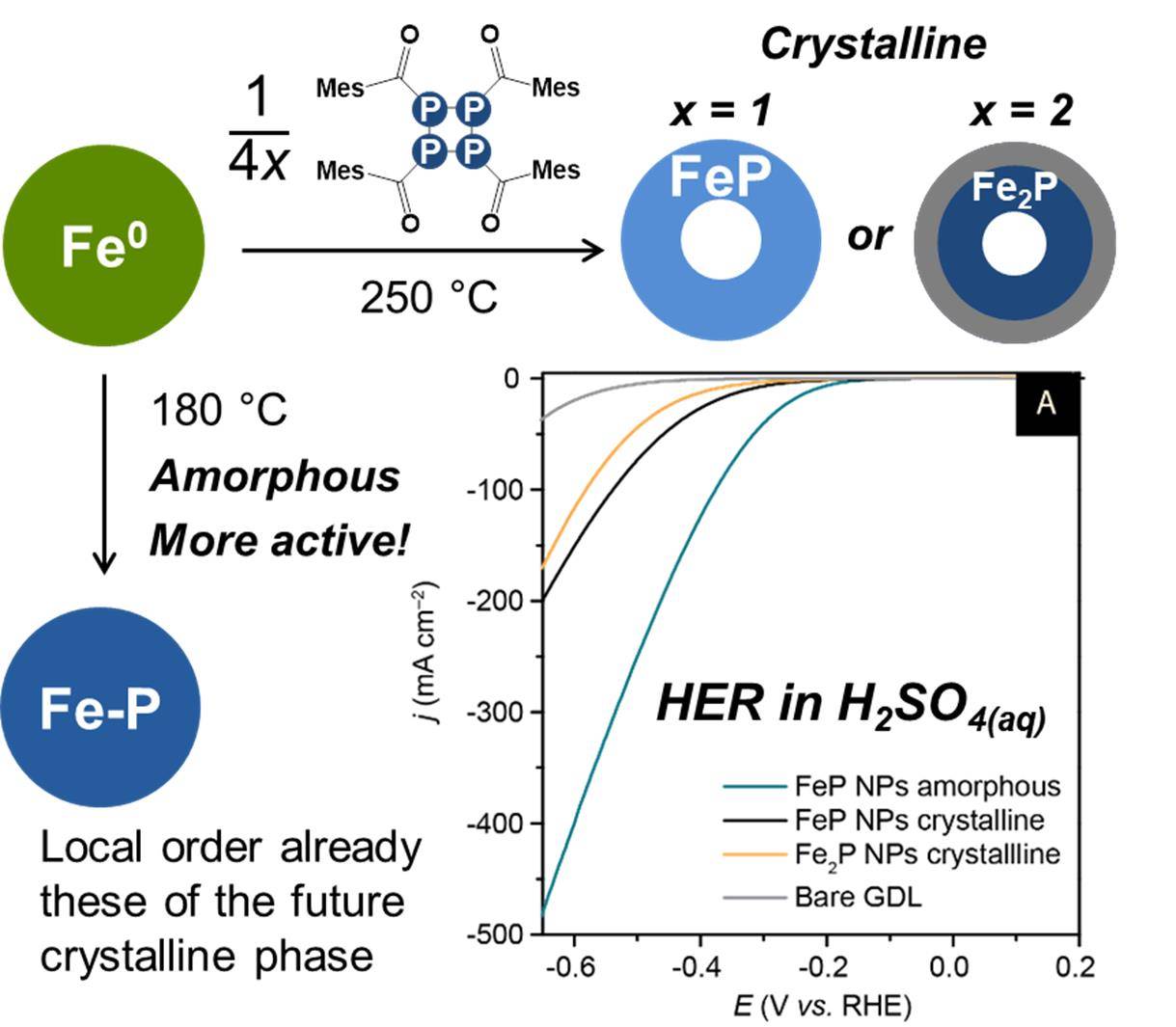 |
Finally mapped out!When we entered the field of oxysulfides in 2015, I found no review on the topic of this rich family of compounds. We took this as a challenge to write it to the community, with a special focus on nanomaterials of course! This review is in Open Access. |
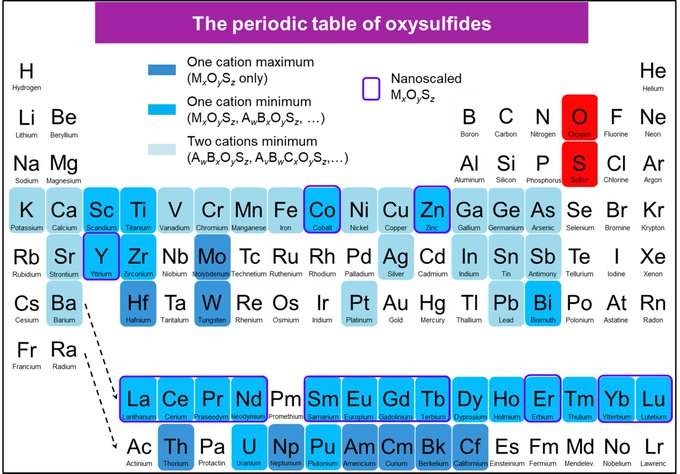 |
Sodium, tell me what you doWe knew for years that sodium ions were mandatory to the formation of colloidal Gd2O2S nanoparticles... but we finally uncovered why! Read the story. |
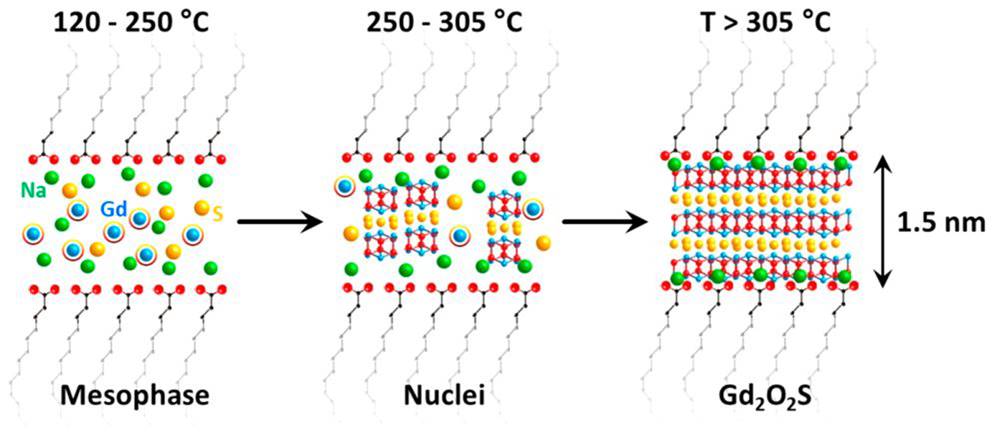 |
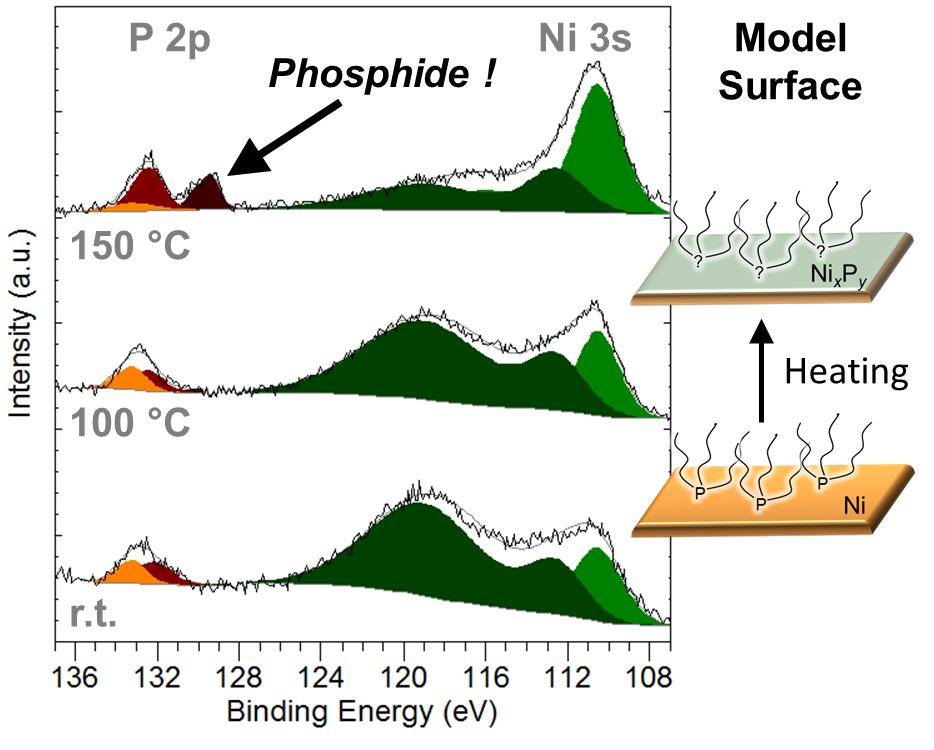
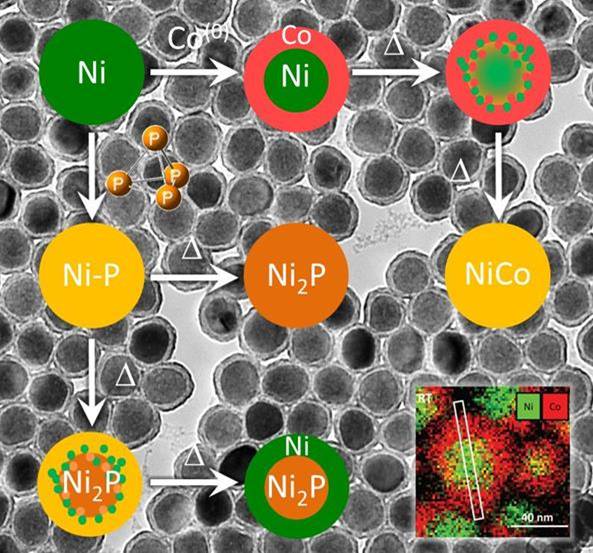
Copper flees the nestMeticulous work at the bench and the microscope finally brought us to understand what happens to core-shell copper-nickel nanoparticles reacted with white phosphorus: copper migrates out! This was achieved within a consortium of teams: two teams at Sorbonne Université, Nicolas Mézailles team at LHFA and Ovidiu Ersen team at IPCMS. Read the paper. |
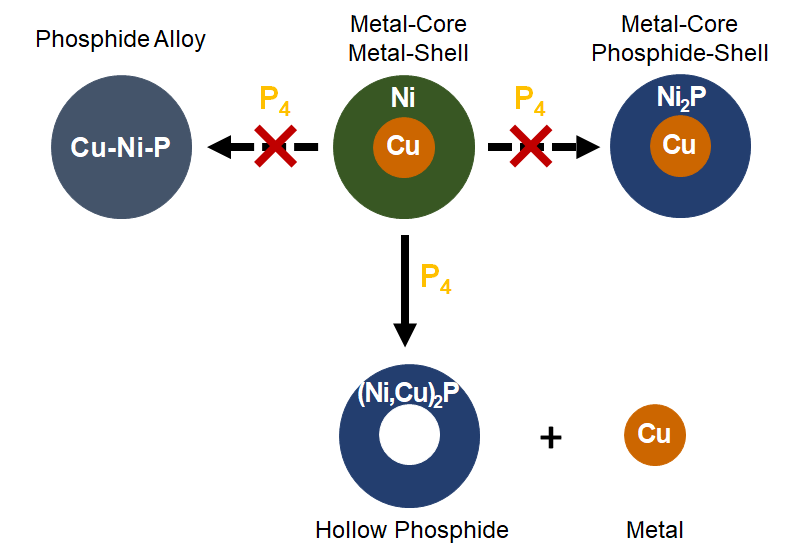 |
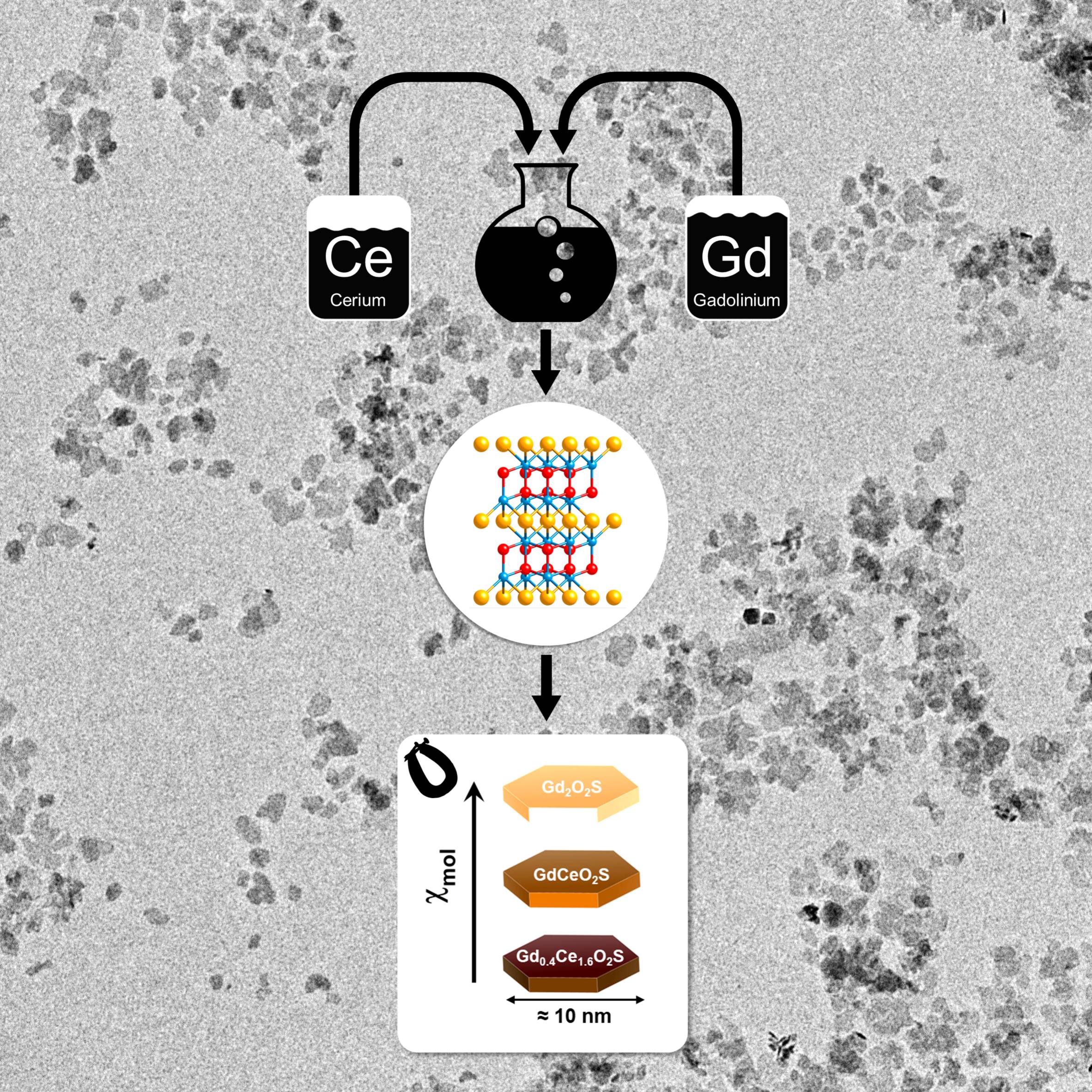
Bandgap: adjustable to your tasteOur study of the bandgap of (Gd,Ce)2O2S nanoparticles, combines synthesis, DFT, PDF, UPS, photoluminescence and more. We show how and why the bandgap is tunable from 4.7 to 2.1 eV, simply by playing on the lanthanide ratio in the solid solution. Read the paper. |
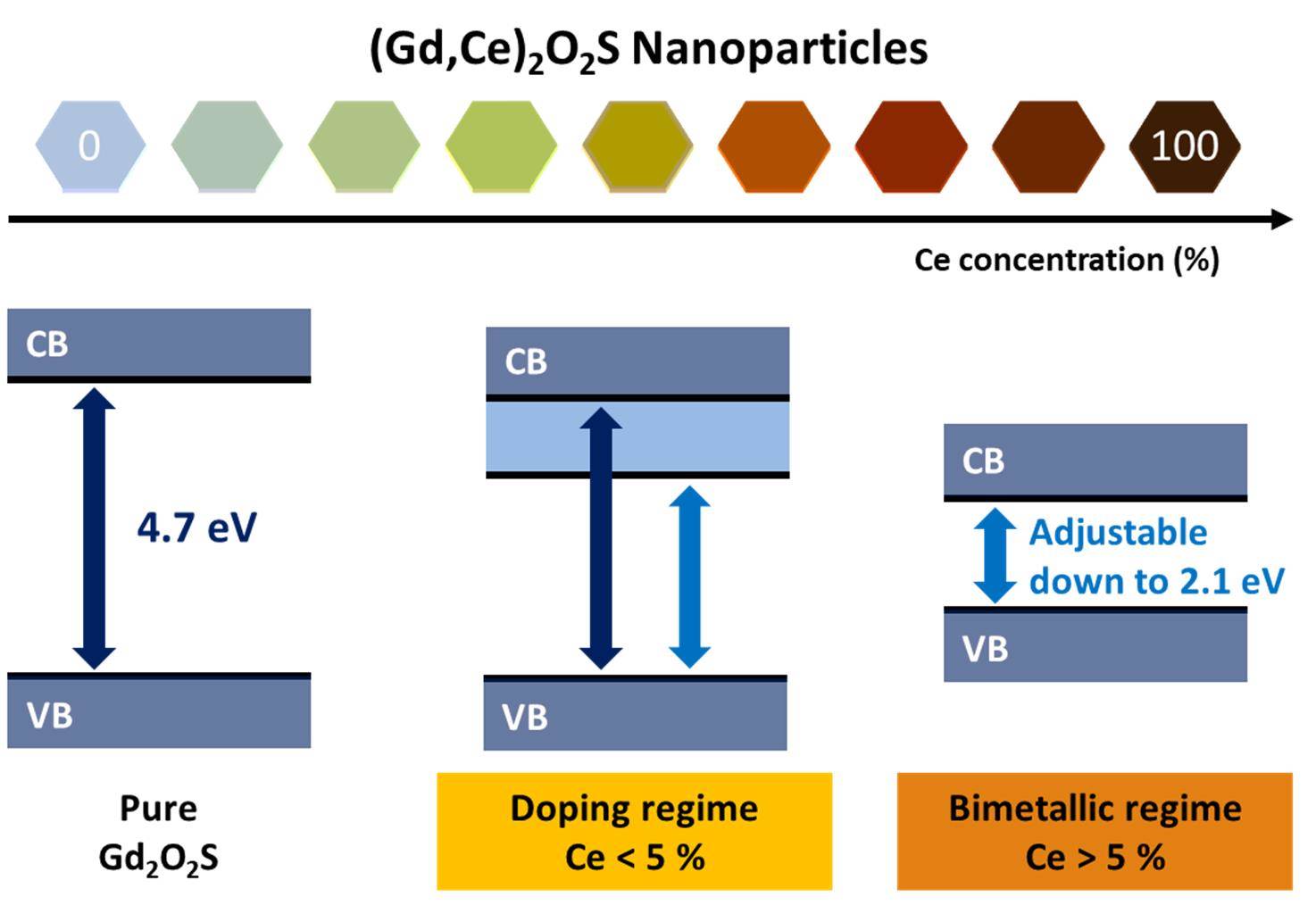 |
Copper and NHC: finally united!This article presents the first report of NHC-stabilized colloidal copper nanoparticles. Using a oxygen-free route, we formed, in one step, trully metallic copper nanoparticles stabilized by NHC ligands. The secret of this good match? An easy-to-reduce [CuMes]n precursor with a more stable NHC-borane adduct. While XPS shows the NHC coordinated to the copper surface, a detailed study using radical traps proposes elementary steps for the reaction mechanism. Read the paper. |
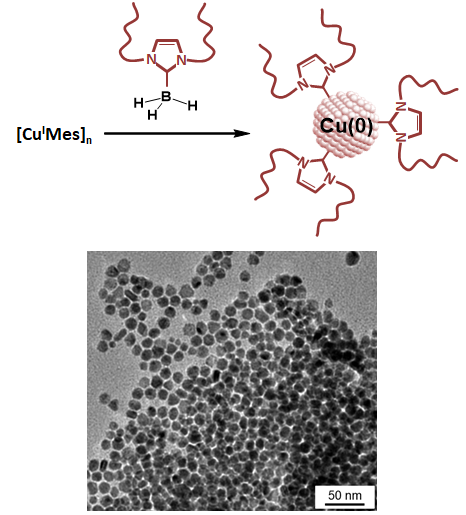 |
Radical or no radical?Serendipity was key for the team entering in the field of silane activation! We found that KC8 acts as a catalyst for the dismutation of PMHS, forming methylsilane. A detailed mechanistic investigation shows that the initation of the reaction goes through a radical pathway. Read the paper. |
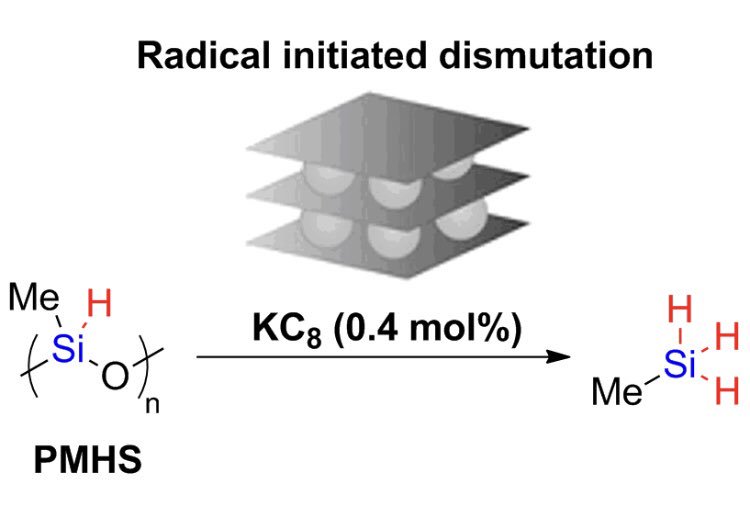 |
Burn them all! (i.e. how to get rid of your ligand buddy)Got a great colloidal synthesis for cute nanoplates, but annoyed by the d*mn ligands that stick around? Just burn them all! under proper conditions of course. Here, we unravel the decomposition path of oleates around metal oxysulfide nanoparticles during inert and oxidizing thermal treatment. Read the paper. |
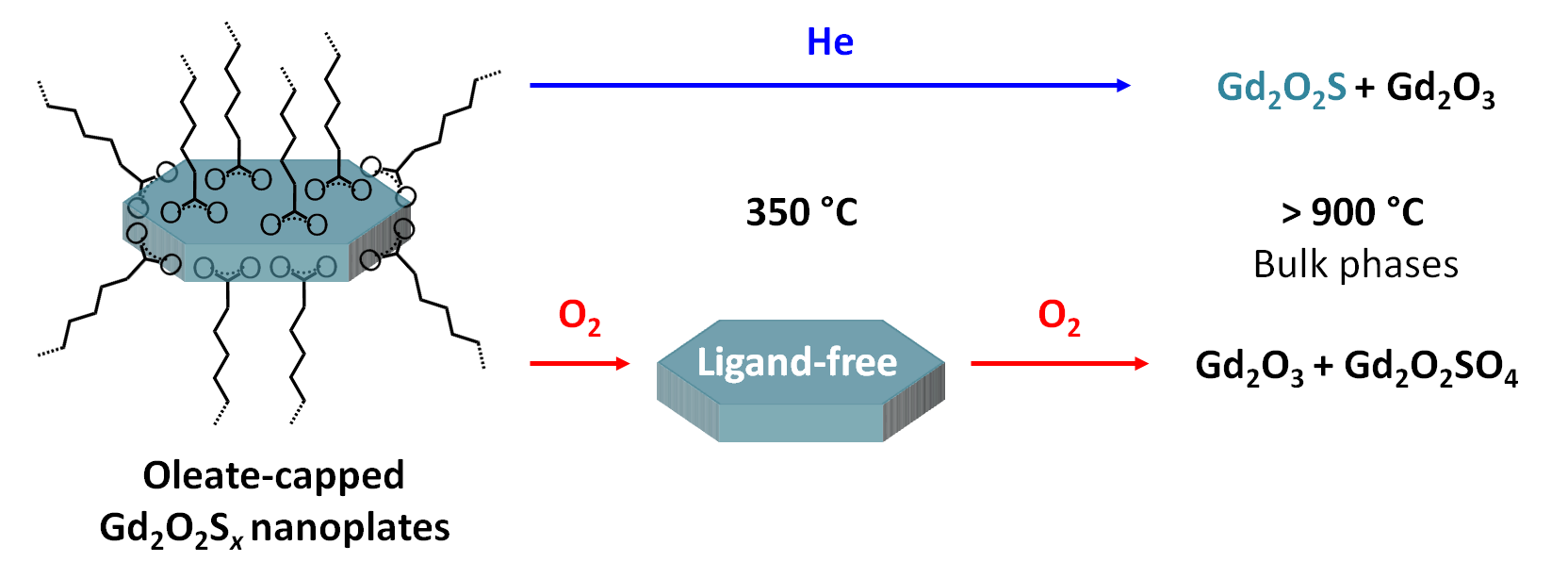 |
Tune your oxysulfide magnetIn this article, we decipher the magnetic properties of (Gd,Ce)2O2S nanoplates prepared by colloidal solution. We show they are directly tuned by the Gd:Ce ratio in the solid solution. Another success for our Labex Matisse project, in collaboration with our colleagues at IMPMC! |
 |
The scaring story of copper nanoparticles vs. phosphinesFunctionalized copper nanoparticles are widely used as catalysts. In this study we investigate hydride-assisted reduction reactions with special focus on the structural evolution of copper nanoparticles in the presence of phosphine and nitrogen-based ligands. Ultrasmall nano-objects are formed as key intermediates to produce catalytically active species in the hydrosilylation of benzaldehyde. Moreover, we found that the strength of the hydride donor is essential for the formation of the active catalysts. Read the paper. |
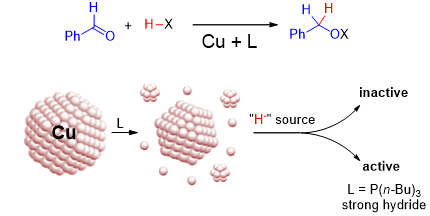 |
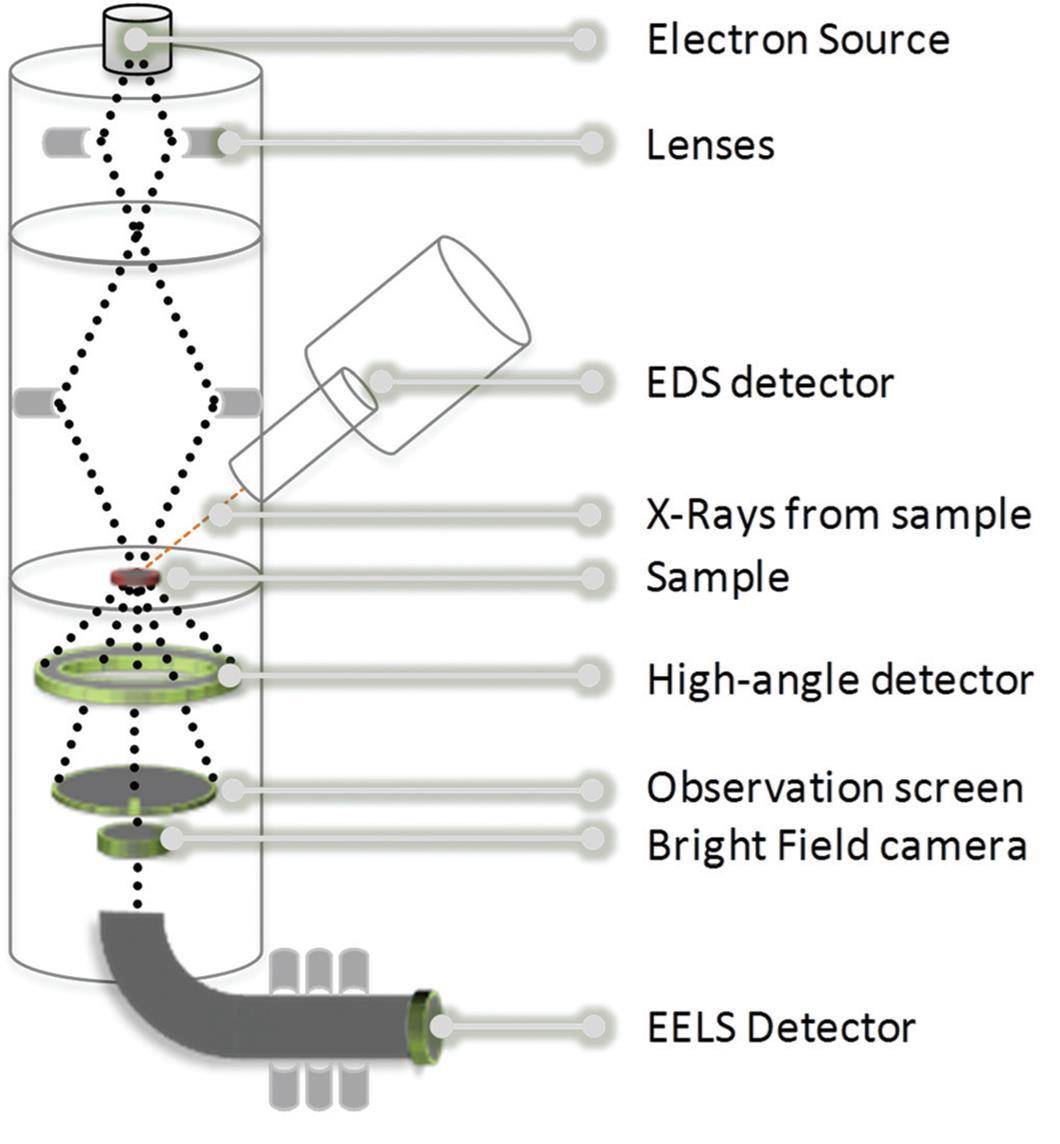
Microscopy and Spectroscopy make the pair!In collaboration with Cecile S. Bonifacio and Judith C. Yang from Pittsburg University, we studied nickel-cobalt nanoparticles exposed to reactive gases. Combination of an ensemble technique (NAP-XPS) and a local one (environmental TEM) was pivotal for describing the nanoparticles transformations. Chem. Eur. J. 2018 (7th Euchems Special Issue) |
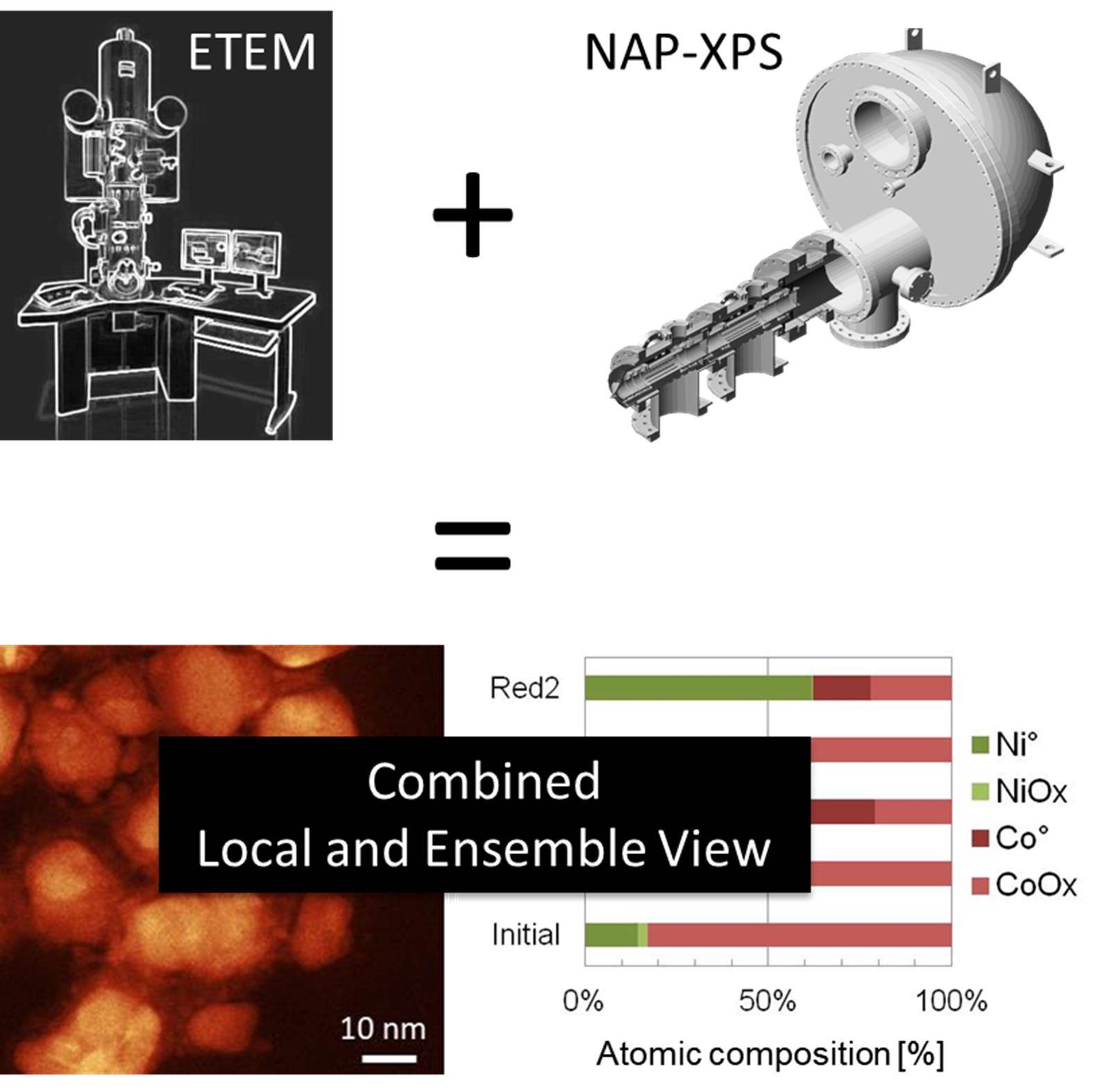 |
Describing inorganic nanoparticles surface reactivityThrough a selection of case studies, this feature article proposes a journey from surface science to nanoparticle design, while illustrating state-of-the-artspectroscopies that help provide a relevant description of inorganic nanoparticles in the context of surface reactivity. Check the paper. |
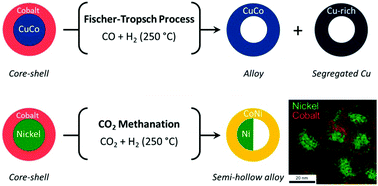 |
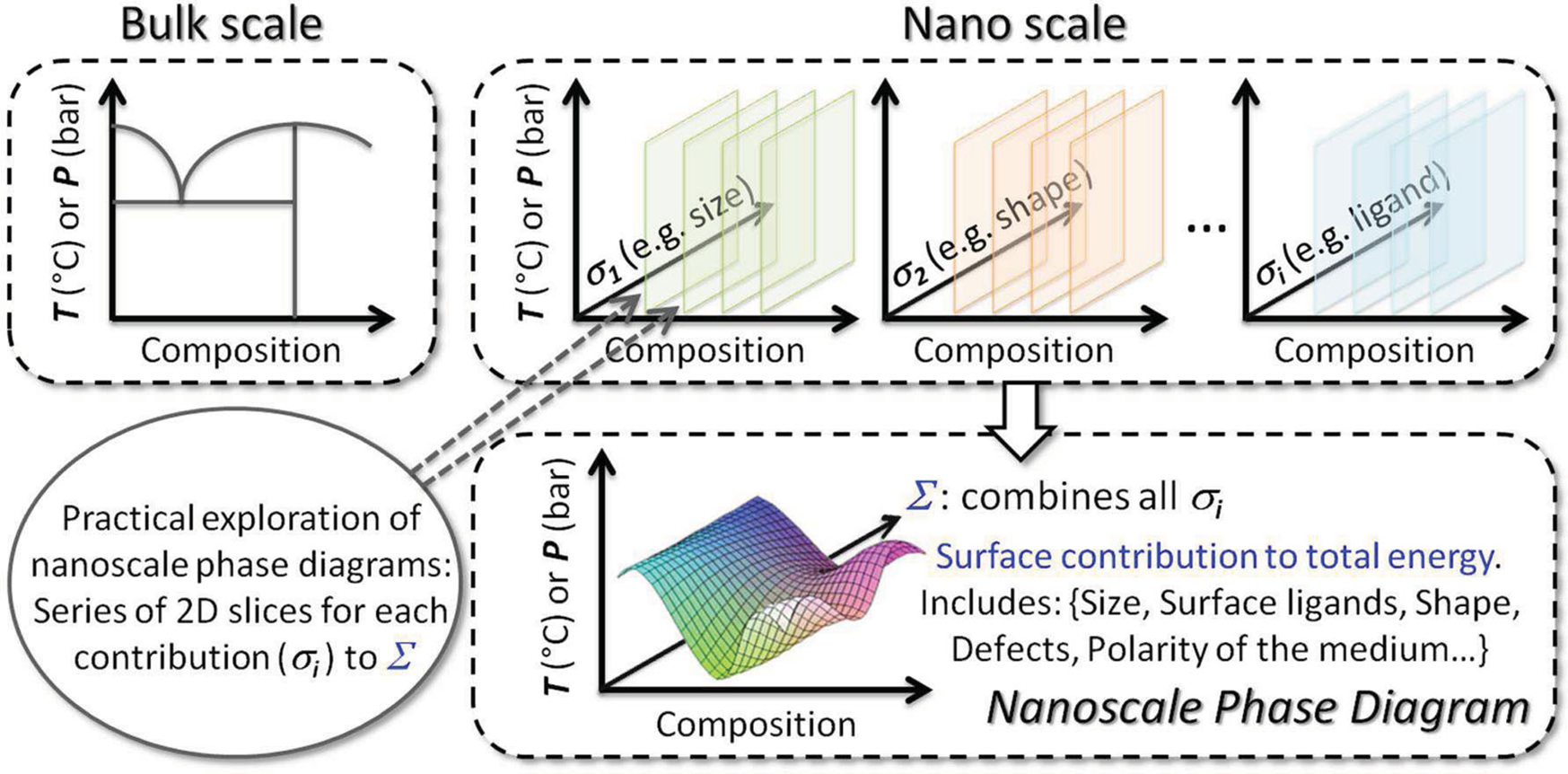
The Design of NanoalloysIn this Personal Account, I discuss the rational design of nanoalloys will be discussed (reactants choice, composition control), in relation with their surface state. Consequences on heterogeneous and homogeneous catalytic reactions, as well as for energy storage and conversion, is illustrated through examples. Check the paper. |
 |
Bimetallic Oxysulfide NanoparticlesOur study on the reactivity of metal oxysulfide nanoparticles was just accepted in Inorganic Chemistry. We synthesized a new family of (Gd,Ce)2O2S nanoparticles and unravelled the relation between their cerium content and their reactivity. Check the paper. |
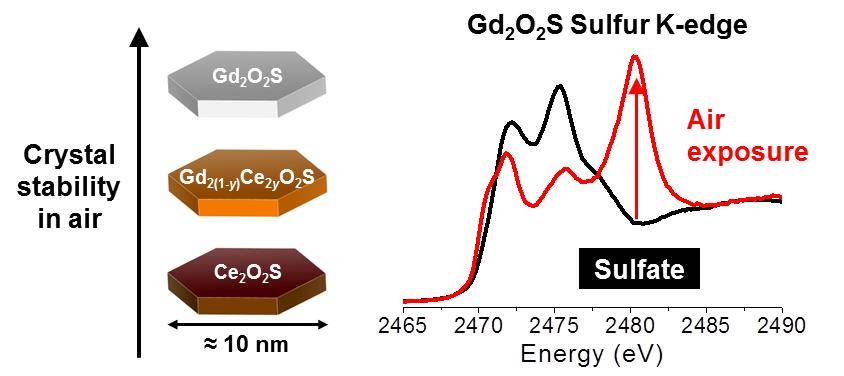 |
Scrutinizing the surface of nanocatalystsWe studied the surface of ruthenium nanoparticles during a catalytic reaction. We showed that the nanoparticles are metallic on the TiO2 support, and that the ratio of C-O and C-H adsorbate species is changing with the temperature. Check the results of this collaboration between Belgium, USA and France. |
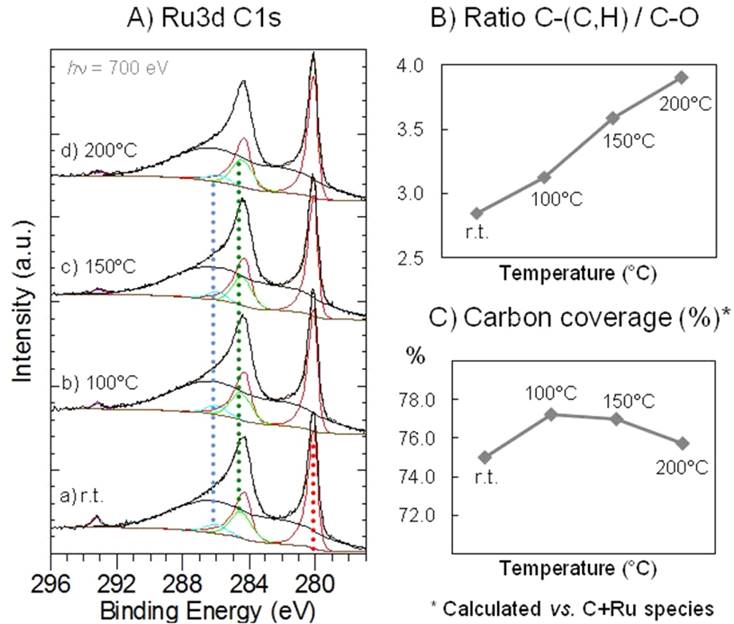 |
Nano-carbides... using a "not-so-soft" synthesisDebora used potassium, a very strong reducing agent, to produce nanoparticles of Vanadium Carbide from liquid VCl4. Check the paper: An expeditious synthesis of early transition metal carbide nanoparticles on graphitic carbons |
 |