(Nano)Chemistry, inside and out
My research is centered on the design, synthesis and applications of nanoparticles using the tools of molecular and materials chemistry.
News and highlights
January 27, 2026: ERC Proof of Concept project acceptedGlad to share the great news: the PRELUDE project was accepted for funding at the ERC Proof of Concept call. We will develop "Precise Deuteration at the Tailored Surface of Nickel Phosphide Nanocatalysts" based on the preliminary results of ERC NanoFLP.
|
 |
January 19, 2026: Podcast interviewThe Fédération Gay-Lussac of Chemistry engineering schools in France hosts a series of podcasts presenting chemists accross the country. I was interviewed in the last episode, and we discussed about the role of nanosciences (in particular, nanochemistry) in research and in society.
|
 |
December 4 , 2025: Journée "Nobels" of Aix Marseille UniversitéDuring this annual one-day conference organized to the benefit of undergrad students, the Nobels of the year are decrypted by experts from the University. I was invited to give the plenary lecture, also opened to the public, at the end of the day.
|
 |
November 28, 2025: Invited conference at GdR NinoI was glad to participate to this exciting workshop "Reactivities", about nanoparticles, their design and their applications, as an invited speaker. It was also a nice opportunity to spend a few days in Paris and catch up with colleagues.
|
 |
October 28, 2025: Award from the Académie des SciencesI am very honored to be the recipient of the Fédération Gay-Lussac/Académie des Sciences award. This award recognizes "Chemistry at the heart of society challenges". (c) Académie des sciences – Mathieu Baumer. |
 |
October 3, 2025: Best oral presentation award: Congrats to Kaltoum!Kaltoum presented her work about low-temperature nickel phosphide at the Meeting on Nanosciences Advances, organized by the C'Nano network. She received the Best Oral Presentation award. Congrats! |
 |
July 9, 2025: Invited conference at SPSSM meetingVery glad to meet and discuss with the international community on solid-state chemistry, in particular related to mixed-anion compounds and their properties. I presented our journey about lanthanide oxysulfide nanoparticles and received highly valuable feedback. Cheers to the organizers! |
 |
More News | Back to top |
Featured Results
Refining the NanoFLP ConceptStill fighting to unveil an authentic NanoFLP, we uncovered an interesting low-temperature behavior of Ni nanoparticles in the presence of well-selected phosphine. This could be interpreted as the formation of a surface frustrated Lewis pair for H-H cleavage... but also as a consequence of phenylacetylene activation. What do you make of this? |
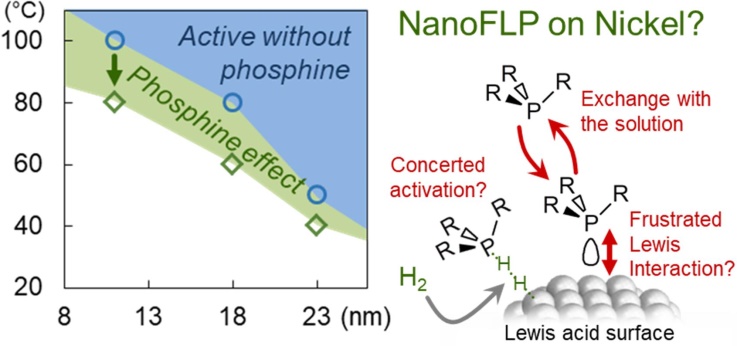 |
Carbon is our friend!Nickel fame for catalysis now extends to a new guy: nickel carbide. In this work, we show that this phase can be used as a catalyst for the hydrgenation of a number of moieties, coming along a greater robustness than the metallic counter-part. Check out our nickel carbide buddy. |
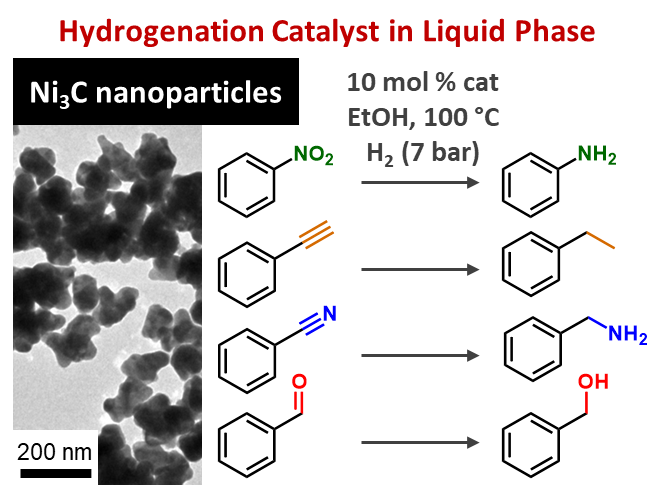 |
Make them saferIt's a small step for the world of nanoparticles, but a big step for our beloved oxysulfides nanoplates: in this work, we propose to evaluate, in a very preliminary approach, some of the interactions of the nanoparticles with macrophage cells. We turned on the lamp (visible light) and monitored the impact in the production of radicals. Read for yourself. |
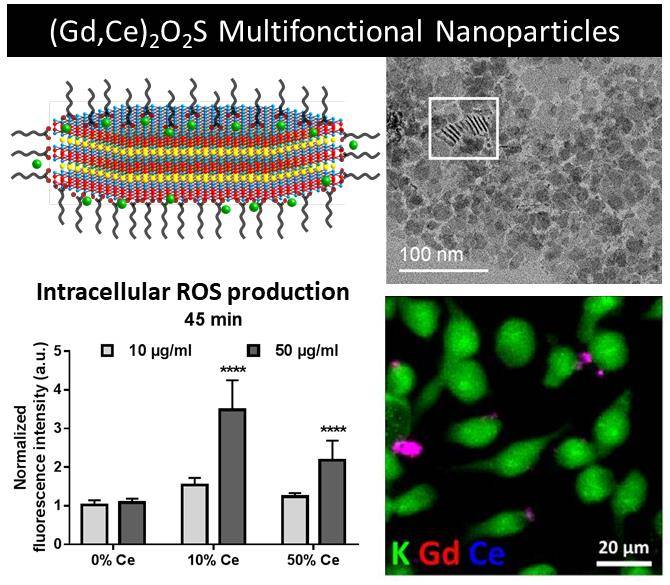 |
Did you say NanoFLP?We have been proposing for a while to use metal nanoparticles as partners in Frustrated Lewis Pairs. Now, we found that nickel-cobalt nanoparticles associated to well-chosen phosphines likely form such a pair, based on the correlation between Si-H bond activation and the Tolman cone angle of the phosphine: only a tiny range of steric hindrance is suitable, and it is related to the silane bulkiness. We believe we nailed it. Make your own opinion. |
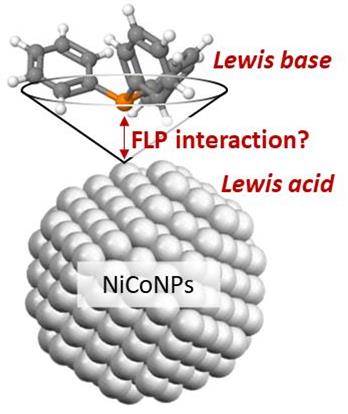 |
Molybdenum mapped outMolybdenum is a key element of the energy transition. X-ray absortion spectroscopy at the L-edge is an amazing way to analyze it in solids, liquids, composites, etc. Here, we wrote some simple guidelines to interpret the data and we provided a consistent series of spectra for well-known, and less-know, Mo compounds. Read our map. |
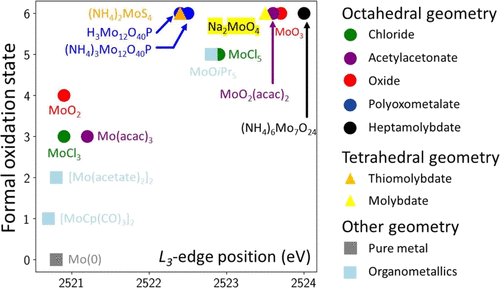 |
More Featured Results | Back to top |




